The pathophysiology is a result of tissue hypoxia and direct toxicity at a cellular level. CO binds to hemoglobin with an affinity of over 200x that of oxygen. This binding results in a shift in the oxygen-dissociation curve to the left, meaning there is increased difficulty in extracting oxygen for tissue use. The increased affinity and tissue hypoxia cannot explain all of the effects of CO toxicity, and reactive oxygen species likely play a role. This is probably mediated through xanthine oxidase.
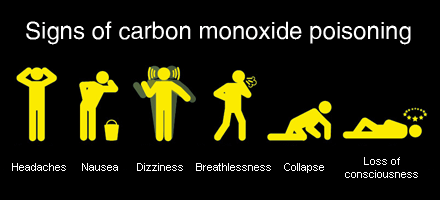
The clinical manifestations of CO poisoning can be subtle. Patients may be mistaken as having influenza (similar symptoms and both peak in winter months), presenting with headache, myalgias, confusion, tachypnea, tachycardia, presyncope and altered LOC. Classically, patients are described as having cherry red lips, though this is an insensitive sign. One retrospective study found the initial diagnosis to be listed as stroke, cardiac ischemia, encephalitis or seizure in multiple cases, highlighting the diagnostic challenge. Other acute symptoms include cardiac chest pain, and this has been described in 30% of cases. This is associated with increased mortality in CO poisoning. Chronic symptoms include the neuropsychiatric syndrome, which presents with personality changes, focal deficits, or cognitive impairment. This occurs with a month following poisoning and can persist for years. The mechanism is not clear, but may be related to myelin destruction from reactive oxygen species.
Because of the non-specific nature of CO poisoning a high index of suspicion is needed to make the diagnosis. The oxygen saturation on pulse oximetry is normal, given the maintained binding of hemoglobin. The diagnosis is made using co-oximetry to measure the amount of carboxyhemoglobin (venous or arterial blood). Amounts of carboxyhemoglobin can be found in several situations:
1-3% carboxyhemoglobin - Normal individuals
under 10% - smokers
Consider the patients history and presentation to determine if the levels are reasonable or as a result of poisoning.
The treatment of CO poisoning is emergency management.
1. ABC's - Many patients will have CO poisining from smoke inhalation. Security of the airway is crucial. Tachypnea, hypoventilation, stridor, facial blistering, burns and edema should prompt intubation and laryngoscopy. Patients can develop airway edema within 24 hours after the inhalation injury following thermal injury, this requires admission and monitoring for respiratory deterioration.
2. Oxygen therapy - all patients should receive high flow 100% oxygen. This results in rapid
improvement in CO levels compared to room air. The half life of CO is 300 minutes, and decreases to 90 minutes when supplimental oxygen is provided.
3. Consider hyperbaric oxygen therapy (HBOT) - Although this therapy is ubiquitous within the literature, the benefit of hyperbaric oxygen is somewhat controversial. There is no doubt that hyperbaric oxygen decreases the half life of oxygen even further than high FiO2 therapy, decreasing the half life to roughly 30 minutes. Whether this extends to improved long-term outcomes is debated. A cochrane review from 2011 found that further RCT's are required to determine whether HBOT should be offered, based on the poor quality of previous studies (see link below for details). Indications are variable, some directives suggests asymptomatic patients with a level of over 40% CO should have HBOT, while others say 25% is a reasonable cutoff. Other reasons to initiate HBOT are:
Pregnancy and CO level over 20%
Decreased LOC or new neurologic deficits
Myocardial ischemia
Acidosis (less than pH 7.1)
4. Isocapneic hyperventilation - this is an experimental treatment that doesn't seem to be widely used in the clinical setting. One study (actually affiliated with anaesthesia at the University of Toronto), showed that in dogs, hyperventilating while providing inhaled CO2 to maintain pH balance resulted in improved removal of CO.
Cochrane review on HBOT