Today in morning report we discussed a case of allopurinol induced Stevens-Johnson syndrome.
Allopurinol is the most commonly used urate-lowering medication in clinical practice, and is most commonly prescribed in the treatment of gout. Adverse reactions involving the skin occur in approximately 2% of patients taking allopurinol.
The drug eruptions can range from mild skin rash to severe reactions including DRESS (drug reaction with eosinophilia and systemic symptoms), and Stevens-Johnson Syndrome / Toxic Epidermal Necrolysis (TEN).
Allopurinol is the drug most responsible for SJS/TEN in Asian countries, and there appears to be a very strong association between HLA-B*58:01 allele and adverse reactions with allopurinol among the Han Chinese population.
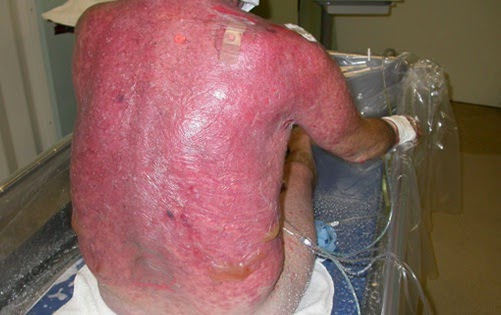 |
| Figure 1: Patient with SJS / TEN - courtesy of Medscape |
What is SJS/TEN
These are severe mucocutaneous reactions most often triggered by medications, but can also be triggered by infectious processes (viral and bacterial).
The distinction between the two is based on severity. SJS typically involves less than 10% of BSA, where as TEN involves >30% of BSA. Both of these conditions often involve the mucous membranes (oral, ocular, or genital), in 90% of cases.
The overall mortality rate for SJS/TEN is approximately 30%, and the mortality is entirely dependent on the amount of BSA involved.
The most common drug culprits include: allopurinol, anti-seizure medications, antibiotics, and NSAIDs.
Clinical Syndrome
Cutaneous involvement - maculopapular rash on the trunk, and often sloughing of the skin "Nikolsky sign".
Mucous membrane involvement (90%)
Fever
Laboratory findings: elevation of LFTs, CBC abnormalities ,electrolyte disturbance.
Management
The cornerstone of management is supportive care (fluid management, wound care) and discontinuation of the underlying precipitant (i.e. allopurinol). A monitored setting is required (ICU or Burn Unit), and consultations from ophthalmology and dermatology are necessary.
There is debate in the literature about the benefits of corticosteroids, IVIG, PLEX, as well as cyclosporine, so discussing the case with your local dermatologist is critical.
References
Association between HLA-B*58:01 allele and severe cutaneous adverse reactions with allopurinol in Han Chinese in Hong Kong. M.L.S. Chiu et al. British Journal of Dermatology. 2012.