Hepatocellular carcinoma (HCC) is a growing concern. Currently, it is listed in the top ten most common cancers worldwide amongst both men and women, and is continuing to increase. The majority of cases are in the setting of viral hepatitis, where hepatitis B makes up nearly 50% of cases. Any causes of cirrhosis can theoretically lead to HCC, and rarely, HCC can develop in the absence of cirrhosis (10%). The burden of hepatitis C has been driving HCV in North America, where HCV related liver cancer is the fastest growing cause of cancer related death in the US.
Patients with HCC tend to present with symptoms of cirrhosis. Jaundice, ascites, GI bleeding and so forth. HCC should be considered in those presenting with acutely decompensated cirrhosis, as it can precipitate worsening liver disease through tumor extension. As an internist, it's also interesting to recognize the various paraneoplastic symptoms of HCC; erythrocytosis, hypoglycemia, sign of leser trelat, hypercalcemia, and diarrhea are recognized phenomenon.
The diagnosis is based on imaging and possible need for biopsy. MRI or CT can be very helpful and when patients have predisposing conditions (HBV), a tumour of 2 cm or greater with typical radiographer pattern in pathognemonic for HCC. Early arterial enhancement and delayed venous washout on a multiphasic scan are suggestive, and indicate tumor vascularity. Tumours less than 1cm. Are difficult to biopsy and require serial scans for monitoring. Intermediate size nodules with atypical pattern should be followed with consideration of biopsy for definitive diagnosis. There are guidelines available on how to approach these nodules which can be very helpful. Alpha-fetoprotein (AFP), when over 500 mcg/L is diagnostic for HCC but intermediate levels can be seen in cirrhosis alone and results need to be taken in context. Levels over 200 mcg/L in the appropriate context have a specificity of over 95%.
Monitoring for HCC is suggested to include liver ultrasound and AFP every 6-12 months in those at risk. This is based on a study published in 2004, where nearly nearly 19,000 Chinese patients with HBV underwent this surveillance. They found a reduction on mortality of 37%. However, A study performed the year prior found no benefit. That being said, this is recommended by the current guidelines. patients that should be monitored include:
1. Asians men over 40 with HBV
2. Asian women over 50 with HBV
3. Patients with HBV and cirrhosis
4. Blacks with HBV
5. Family history of HCC and HBV
6. Any patient with cirrhosis
It is less clear when to start screening Caucasians with HBV, and some recommend initating at similar times to the Asian population, despite a lower risk of developing HCC. It may less cost effective to screen in those with cirrhosis from alcohol given a lower risk of HCC, but the epidemiology is not well described. Attached below is a nice NEJM review which contains many of the resources with above information.
HCC review
Thursday, September 26, 2013
Wednesday, September 25, 2013
Hypoglycemia
Hypoglycemia is dangerous and common event for many patients with diabetes. Although it is more common in DM1, many patients with DM2 will experience this problem. The Diabetes Control and Complications Trial (DCCT) identified hypoglycemia as a major concern, causing symptoms approximately 2 times a week in those with DM1. As patients live longer with DM2, they also develop an increased frequency of hypoglycemia, where 25% of patients using insulin for over 5 years experience this problem regularly. Much of our focus when caring for patients with diabetes is lowering their blood sugar in the hopes of preventing complications. Microvascular complications are reduced by improving glycemic control in both patients with DM1 and DM2, making it the focus of diabetes care. However, randomized trials targeting lower HbA1c levels (ex. ACCORD), found increased mortality in those randomized to tighter glucose control. Although not directly proven, this is likely a result of increased hypoglycemia. This highlights that severe symptoms can occur as a result of hypoglycemia. A study published in Diabetes Care found 100% of patients with DM1 experienced severe hypoglycemia at some point, requiring medical intervention. Recognizing the problem is an opportunity for prevention. Many diabetics with recurrent episodes lose the ability to sense hypoglycemia, putting them at risk for lower levels and additional risks. Symptoms can be broken down into two categories:

1. Neurogenic symptoms - tremor, palpitations, arousal, sweating, hunger (mediated through catecholamine and acetylcholine mechanisms).
2. Neuroglycopenic symptoms - cognitive impairment, coma, seizure and death.
Patient who experience recurrent hypoglycemia are at increased risk for developing hypoglycemia unawareness. This is when, despite hypoglycemia, patients lack symptoms to suggest a problem. Often the first symptom in these patients is confusion, which makes it hard for them to respond appropriately with increased sugar intake. Hypoglycemia unawareness can lead to hypoglycemia associated autonomic failure (HAAF), a form of autonomic insufficiency from recurrent hypoglycemia. In HAAF, patients are unable to mount an appropriate adrenergic response to low blood glucose. HAAF is associated with a significant risk of severe hypoglycemia (25x those without). If glucose levels improve and are maintained, patients will regain the function of their catecholamine axis in several weeks.
Definitions of hypoglycemia are different whether or not a diagnosis of diabetes is present.In those with diabetes any glucose less than 3.9 mmol is considered low. In those without diabetes the guidelines state that patients must have whipples triad:
1. Symptoms that may be explained by hypoglycemia
2. Documented hypoglycemia at the time of symptoms
3.Improvement of symptoms after taking glucose
There are many causes of hypoglycemia, and the approach is different in those with and without diabetes. The most common cause of low glucose is druge related. Several categories exist and can be separated by either "sick patients/medicated" or "well patients":
"Sick/medicated patient"
1. Drugs-insulin, alcohol
2. Critical illness- sepsis
3. Organ failure- renal/liver failure
4. Cortisol deficiency
5. Non-islet cell tumours
"Well patient"
1. Endogenous overproduction of insulin- insulinoma, nesidioblastosis
2. Autimmune- antibodies against endogenous insulin
3. Use of secretagogue- accidental, serruptitious etc.
More details regarding investigations for hypoglycemia and management of this illness in patients with diabetes can be found in the guidelines linked below.
Hypoglycemia guidelines

1. Neurogenic symptoms - tremor, palpitations, arousal, sweating, hunger (mediated through catecholamine and acetylcholine mechanisms).
2. Neuroglycopenic symptoms - cognitive impairment, coma, seizure and death.
Patient who experience recurrent hypoglycemia are at increased risk for developing hypoglycemia unawareness. This is when, despite hypoglycemia, patients lack symptoms to suggest a problem. Often the first symptom in these patients is confusion, which makes it hard for them to respond appropriately with increased sugar intake. Hypoglycemia unawareness can lead to hypoglycemia associated autonomic failure (HAAF), a form of autonomic insufficiency from recurrent hypoglycemia. In HAAF, patients are unable to mount an appropriate adrenergic response to low blood glucose. HAAF is associated with a significant risk of severe hypoglycemia (25x those without). If glucose levels improve and are maintained, patients will regain the function of their catecholamine axis in several weeks.
Definitions of hypoglycemia are different whether or not a diagnosis of diabetes is present.In those with diabetes any glucose less than 3.9 mmol is considered low. In those without diabetes the guidelines state that patients must have whipples triad:
1. Symptoms that may be explained by hypoglycemia
2. Documented hypoglycemia at the time of symptoms
3.Improvement of symptoms after taking glucose
There are many causes of hypoglycemia, and the approach is different in those with and without diabetes. The most common cause of low glucose is druge related. Several categories exist and can be separated by either "sick patients/medicated" or "well patients":
"Sick/medicated patient"
1. Drugs-insulin, alcohol
2. Critical illness- sepsis
3. Organ failure- renal/liver failure
4. Cortisol deficiency
5. Non-islet cell tumours
"Well patient"
1. Endogenous overproduction of insulin- insulinoma, nesidioblastosis
2. Autimmune- antibodies against endogenous insulin
3. Use of secretagogue- accidental, serruptitious etc.
More details regarding investigations for hypoglycemia and management of this illness in patients with diabetes can be found in the guidelines linked below.
Hypoglycemia guidelines
Thursday, September 19, 2013
Suspected overdose
Paracelsus, the Renaissance physician said that "the dose determines that a thing is not a poison", recognizing that most things in excess can be harmful. In the US there are over 2.4 million documented toxin exposures reported to poison control centres, the majority of which occur in children. The leading agents causing death are analgesics, antidepressants, cardiovascular medications, stimulants and illicit drugs.
Your approach to the poisoned patient is two pronged, containing a diagnosis and a treatment are in parallel. The treatment consists of ABC's, D (decontamination/DONT antidotes), E (enhanced elimination), F focused therapy and G (get help). The diagnostic are requires a history, physical exam, search for toxidrome and diagnostic testing.
 History is key. How much? When? What? Why? All these can be helpful and alter your approach to the patient. Often patients cant name the medications they're taking or they do so incorrectly (Tylenol vs aspirin). If it is not available, obtaining information from family, work, family physician may be helpful. Specific symptoms to consider include:
History is key. How much? When? What? Why? All these can be helpful and alter your approach to the patient. Often patients cant name the medications they're taking or they do so incorrectly (Tylenol vs aspirin). If it is not available, obtaining information from family, work, family physician may be helpful. Specific symptoms to consider include:
protracted coughing - hydrocarbon exposure
inability to swallow/drooling - costic ingestion
hematemesis with iron ingestion
persistent seizures with INH overdose
decreased LOC with carbon monoxide
There are a number of pneumonics to help you remember toxidromes. Below is an article that contains an extensive list. Things to focus on include vital signs (HR, BP, temp, GCS, seizure), pupils (meiosis, mydriasis), odour (garlic-organophosphate, wintergreen - methylsalicylates, almond - cyanide), neurologic exam (rigidity/clonus - SS/NMS), skin (rash, diaphoresis, track markrs, fentanyl patches).
Preliminary labs will include routines, plus toxicology screen, osmolal gap and anion gap. Toxicology can be performed on serum and urine, where urine test tend to identify metabolites and will have positive results for longer duration compared to serum. Quantitative levels should only be performed if it suggests higher toxicity and will alter management. Levels can be performed on: tylenol, ASA, Li, Fe, CO, digoxin, anticonvulsants, toxic alcohols and theophylline. Other aspects of urine testing include any visual change, such as a change in UV light with ethylene glycol, orange with rifampin, pink with ampicillin, green with copper or methylene blue. Microscopy for calcium oxalate crystals also suggests ethylene glycol poisoning (see link below). Imaging tests may include CXR/AXR to look for iron or ingested street drugs (body packing with cocaine). Drugs causing pneumonitis or pulmonary edema can be remembered as MOPS (methadone, opioids, phenobarbitol/phosgene, salicylates).
Today we mentioned that "one pill can kill", although this tends to be targeted more towards children it highlights the potential toxicity of these medications. A top ten list of these drugs includes, but is not limited to:
1. TCA
2. antipsychotics
3. antimalarials
4. anti-arrhythmics
5. camphor
6. oral hypoglycemics - sulfonylureas
7. opioids
8. theophylline/podophylline
9. salicylates
10. calcium channel blockers
Calling poison control is always the right thing to do. You will be put in touch with an experienced nurse and have access to a clinical toxicologist if necessary. They also document each case and follow-up on the patient. For a more detailed approach to therapy see the article below.
Approach to unknown overdose
calcium oxalate cyrstals
fluorescent urine in ethylene glycol ingestion
Your approach to the poisoned patient is two pronged, containing a diagnosis and a treatment are in parallel. The treatment consists of ABC's, D (decontamination/DONT antidotes), E (enhanced elimination), F focused therapy and G (get help). The diagnostic are requires a history, physical exam, search for toxidrome and diagnostic testing.
protracted coughing - hydrocarbon exposure
inability to swallow/drooling - costic ingestion
hematemesis with iron ingestion
persistent seizures with INH overdose
decreased LOC with carbon monoxide
There are a number of pneumonics to help you remember toxidromes. Below is an article that contains an extensive list. Things to focus on include vital signs (HR, BP, temp, GCS, seizure), pupils (meiosis, mydriasis), odour (garlic-organophosphate, wintergreen - methylsalicylates, almond - cyanide), neurologic exam (rigidity/clonus - SS/NMS), skin (rash, diaphoresis, track markrs, fentanyl patches).
Preliminary labs will include routines, plus toxicology screen, osmolal gap and anion gap. Toxicology can be performed on serum and urine, where urine test tend to identify metabolites and will have positive results for longer duration compared to serum. Quantitative levels should only be performed if it suggests higher toxicity and will alter management. Levels can be performed on: tylenol, ASA, Li, Fe, CO, digoxin, anticonvulsants, toxic alcohols and theophylline. Other aspects of urine testing include any visual change, such as a change in UV light with ethylene glycol, orange with rifampin, pink with ampicillin, green with copper or methylene blue. Microscopy for calcium oxalate crystals also suggests ethylene glycol poisoning (see link below). Imaging tests may include CXR/AXR to look for iron or ingested street drugs (body packing with cocaine). Drugs causing pneumonitis or pulmonary edema can be remembered as MOPS (methadone, opioids, phenobarbitol/phosgene, salicylates).
Today we mentioned that "one pill can kill", although this tends to be targeted more towards children it highlights the potential toxicity of these medications. A top ten list of these drugs includes, but is not limited to:
1. TCA
2. antipsychotics
3. antimalarials
4. anti-arrhythmics
5. camphor
6. oral hypoglycemics - sulfonylureas
7. opioids
8. theophylline/podophylline
9. salicylates
10. calcium channel blockers
Calling poison control is always the right thing to do. You will be put in touch with an experienced nurse and have access to a clinical toxicologist if necessary. They also document each case and follow-up on the patient. For a more detailed approach to therapy see the article below.
Approach to unknown overdose
calcium oxalate cyrstals
fluorescent urine in ethylene glycol ingestion
Wednesday, September 18, 2013
Alcohol use - keeping an eye open
The complications of alcohol use are many. We are very good at listing the extensive number of possible problems that come along with alcohol use; nutritional deficiencies, myopathy, cognitive impairment, cirrhosis, cardiomyopathy, etc. however we are often fail to characterize the problem. Physicians underestimate the amount of alcohol ingested and when its identified, fail to capitalize on opportunities for rehabilitation. A US study found that only 48% of patients identified as having problem drinking were asked to follow-up, leaving the majority without ongoing monitoring. Patients may fail to have recognizable signs of cirrhosis despite significant liver injury, requiring probing questions to first identify the problem.
 There are many tools for evaluating alcohol abuse. The most commonly used tool is the CAGE questionnaire. This method is great because of its simplicity and ease of administration, but fails to determine long term or changes in drinking patterns.It also lacks a quantitative component, which can be helpful to gauge severity of disease. A meta-analysis evaluating its use found the test to be more useful in inpatients compared to ambulatory assessment with a sensitivity of 87% and specificity of 90% for >2 components. Other tests do exist.
There are many tools for evaluating alcohol abuse. The most commonly used tool is the CAGE questionnaire. This method is great because of its simplicity and ease of administration, but fails to determine long term or changes in drinking patterns.It also lacks a quantitative component, which can be helpful to gauge severity of disease. A meta-analysis evaluating its use found the test to be more useful in inpatients compared to ambulatory assessment with a sensitivity of 87% and specificity of 90% for >2 components. Other tests do exist.
The alcohol use disorders identification test (AUDIT) is a longer test with higher sensitivity and specificity (96% and 97%). This was developed by the World Health Organization, and as a result was created for international use, validated in multiple languages. Of its 10 questions, it covers consumption, consequences and dependence issues, covering more areas than shorted questionnaires. Values of 8 and 20 are important to remember, being markers of harmful alcohol use and dependence.
Other markers of alcohol use which are mentioned on the wards and in the literature are macrocytosis and GGT levels. Unfortunately, these tests have limited sensitivity and specificity. A study in Hepatology from 1984 found GGT did offer some prognostic information in patients with acoholic cirrhosis, where only 60% of those with a level of 100 IU survived to 1 year.
An additionally proposed test for alcohol consumption is measurement of carbohydrate deficient transferrin. Transferrin circulates in our bodies in glycosylated forms. There is a variety of sugars attached to transferrin in different amounts. Alcohol consumption results in reduction of carbohydrate numbers on the transferrin molecule, something that can be detected in the blood. Studies have found that patients with low AUDIT scores are unlikely to have carbohydrate deficient transferrin. The test characteristics have been proposed to be better than that of GGT. I am not familiar with the cost of this assay and have never heard of anyone ordering this test.
All patients admitted with alcohol related complications, and more importantly those seen as outpatients with risk factors for alcohol abuse should be screened. Multiple methods exist, but when you have time the AUDIT form seems like an appropriate choice. See additional details below.
AUDIT score from BMJ best practices
 There are many tools for evaluating alcohol abuse. The most commonly used tool is the CAGE questionnaire. This method is great because of its simplicity and ease of administration, but fails to determine long term or changes in drinking patterns.It also lacks a quantitative component, which can be helpful to gauge severity of disease. A meta-analysis evaluating its use found the test to be more useful in inpatients compared to ambulatory assessment with a sensitivity of 87% and specificity of 90% for >2 components. Other tests do exist.
There are many tools for evaluating alcohol abuse. The most commonly used tool is the CAGE questionnaire. This method is great because of its simplicity and ease of administration, but fails to determine long term or changes in drinking patterns.It also lacks a quantitative component, which can be helpful to gauge severity of disease. A meta-analysis evaluating its use found the test to be more useful in inpatients compared to ambulatory assessment with a sensitivity of 87% and specificity of 90% for >2 components. Other tests do exist.The alcohol use disorders identification test (AUDIT) is a longer test with higher sensitivity and specificity (96% and 97%). This was developed by the World Health Organization, and as a result was created for international use, validated in multiple languages. Of its 10 questions, it covers consumption, consequences and dependence issues, covering more areas than shorted questionnaires. Values of 8 and 20 are important to remember, being markers of harmful alcohol use and dependence.
Other markers of alcohol use which are mentioned on the wards and in the literature are macrocytosis and GGT levels. Unfortunately, these tests have limited sensitivity and specificity. A study in Hepatology from 1984 found GGT did offer some prognostic information in patients with acoholic cirrhosis, where only 60% of those with a level of 100 IU survived to 1 year.
An additionally proposed test for alcohol consumption is measurement of carbohydrate deficient transferrin. Transferrin circulates in our bodies in glycosylated forms. There is a variety of sugars attached to transferrin in different amounts. Alcohol consumption results in reduction of carbohydrate numbers on the transferrin molecule, something that can be detected in the blood. Studies have found that patients with low AUDIT scores are unlikely to have carbohydrate deficient transferrin. The test characteristics have been proposed to be better than that of GGT. I am not familiar with the cost of this assay and have never heard of anyone ordering this test.
All patients admitted with alcohol related complications, and more importantly those seen as outpatients with risk factors for alcohol abuse should be screened. Multiple methods exist, but when you have time the AUDIT form seems like an appropriate choice. See additional details below.
AUDIT score from BMJ best practices
Friday, September 13, 2013
Malignancy and diarrhea
Infectious causes dominate the majority of causes for acute diarrhea, and its often what we first consider based on investigations, and possible treatments. However, with diarrhea lasting more than 2 weeks, persistent and chronic diarrhea are considered, and after the infectious group we have to think about other classes of disease.
Malignancy is an uncommon but ominous cause of diarrhea. This association can result from several ways, and categorizing the type of diarrhea can be helpful. Typical groups include the following:
1. Watery diarrhea - secetory vs. osmotic
2. Fatty diarrhea
3. Inflammatory diarrhea
Watery diarrhea can be identified on history, where patients tend to have large volume, frequent stools, without solid material. Patients will often describe diarrhea despite not eating, and having to wake in the night to have a bowel movement in secretory/osmotic causes. Although its often not necessary, the stool osmolality gap can be calculated to differentiate between osmotic and secretory where a gap of greater than 125 suggests a osmotic cause. Cancers causing secretory diarrhea include the islet cell tumours which secrete hormones including: gastrinoma, glucagonoma, vasoactive intestinal peptides tumours, and pancreatic polypeptide tumours. Increased seratonin is also felt to be the cause of secretory diarrhea that occurs in carcinoid syndrome. Patients with medullary thyroid cancer complain of diarrhea as a ommon symptom in metastatic disease present in 40% of patients. The mechanism is controversial, but calcitonin production is thought to play a role. The diarrhea in this disease was clasically considered a secretory diarrhea, though some studies have found an increased electrolyte gap in the stool suggesting an osmotic cause. Lymphoma (when present in the gut) and villous adenomas of the bowel are also felt to produce a secretory pattern of diarrhea.
Fatty diarrhea is diagnosed using a 72h quantitative fecal fat test. Additional sudan staining can confirm the presence of fat in the stool. Patients will often say that they have greasy, oily stool that doesnt go down the toilet after flushing because it floats. Malabsorption from multiple causes can present like this, the most common likely being celiac disease. However, again malignancy can cause a similar presentation. Pancreatic cancer that results in exocrine dysfuntion can impair fat digestion in the bowel and lead to fatty diarrhea. Biliary obstruction from pancreatic cancer will decrease the release of bile salts in to the bowels and again impair fat absorption as a result, contributing to diarrhea. Somatostatin secreting tumours are documented as causing fatty diarrhea, these are islet cell tumours that produce octreotide, inhibit pancreatic function and bicarbonate release, which results in fat malabsorption.
Many patients with cancer are on chemotherapy and are therefore immunosuppressed. Diarrhea may be a direct result of chemotherapeutic agents, or newly acquired infection. Our first focus of care in diarrhea should be to rule out infection and avoid exacerbating medications given these things can be life threatening and are treatable.
Thursday, September 12, 2013
Renal artery stenosis
In hospitalized patients acute kidney injury is largely attributed to pre-renal disease, with additional renal and post renal causes being much less likely. Although a trial of fluids is usually tried in the majority of patients its important to recognize when this therapy is failing and other causes of renal should be considered. Renal artery stenosis (RAS) is an uncommon cause of chronic hypertension (less than 1%), but is more likely to be causative in acute refractory cases. In general, RAS can be broken into two classes:

1. Atherosclerotic stenosis (90%)
2. Fibromuscular dysplasia (10%)
Atherosclerotic disease is much more common than FMD given the presence of CAD, DMII, HTN, dyslipidemia and smoking prevalence. It is a progressive disease which usually results in small kidneys and CKD. Fibromuscular dysplasia is an abnormality of the intimal, medial and adventitial layers of the blood vessels of unclear cause. It results in narrowing of the renal arteries, typically at the distal third. Risk factors include being female and HTN prior to age 50. These patients can develop other complications, including artery dissection or thrombosis (most common with intimal involvement).
The pathophysiology is related to poor renal perfusion leading to activation of the renin-angiotensin system, impacting sodium homeostasis, vasodilatory factors and results in renal hypertension. Clinical clues to suspect renal vascular disease in patients with CKD and HTN include:
1. Worsening kidney function of 30% in started and ACEi
2. Systolic/Diastolic renal bruit
3. Onset HTN after 55
4. Flash pulmonary edema
5. Asymmetry in renal size >1.5 cm
Testing for these disease used to involve a captopril renal scan where differences in renal perfusion were examined following administration of an ACEi. However, this is no longer done and has largely been replaced by imaging. Ultrasound with arterial dopplers are helpful, and certain flow velocities through the artery can predict the degree of stenosis. This does however require an experienced radiologists and can be difficult to get appropriate view. CT angiography and MRI are useful tests, but are less likely to image distal vessels appropriately.
All patients are recommended to undergo medical therapy, which involves modification of CAD risk factors, including smoking cessation. ACEi will preferentially vasodilate the efferent renal artery, counteracting the natural response to improving GFR in patients with afferent renovascular disease. The addition of an ACEi can decrease GFR and worsen AKI. That being said, they can still be used in this disease and one must consider the clinical context. This is more problematic inpatients with atherosclerotic disease as opposed to FMD.
Renal artery angioplasty has not been shown to be superior to medical therapy in a meta-analysis published in the American Heart Journal in 2011. However, patients with refractory hypertension and flash pulmonary edema, may be considered for angioplasty. See this NEJM review on RAS for additional details.
Renal artery stenosis review

1. Atherosclerotic stenosis (90%)
2. Fibromuscular dysplasia (10%)
Atherosclerotic disease is much more common than FMD given the presence of CAD, DMII, HTN, dyslipidemia and smoking prevalence. It is a progressive disease which usually results in small kidneys and CKD. Fibromuscular dysplasia is an abnormality of the intimal, medial and adventitial layers of the blood vessels of unclear cause. It results in narrowing of the renal arteries, typically at the distal third. Risk factors include being female and HTN prior to age 50. These patients can develop other complications, including artery dissection or thrombosis (most common with intimal involvement).
The pathophysiology is related to poor renal perfusion leading to activation of the renin-angiotensin system, impacting sodium homeostasis, vasodilatory factors and results in renal hypertension. Clinical clues to suspect renal vascular disease in patients with CKD and HTN include:
1. Worsening kidney function of 30% in started and ACEi
2. Systolic/Diastolic renal bruit
3. Onset HTN after 55
4. Flash pulmonary edema
5. Asymmetry in renal size >1.5 cm
Testing for these disease used to involve a captopril renal scan where differences in renal perfusion were examined following administration of an ACEi. However, this is no longer done and has largely been replaced by imaging. Ultrasound with arterial dopplers are helpful, and certain flow velocities through the artery can predict the degree of stenosis. This does however require an experienced radiologists and can be difficult to get appropriate view. CT angiography and MRI are useful tests, but are less likely to image distal vessels appropriately.
All patients are recommended to undergo medical therapy, which involves modification of CAD risk factors, including smoking cessation. ACEi will preferentially vasodilate the efferent renal artery, counteracting the natural response to improving GFR in patients with afferent renovascular disease. The addition of an ACEi can decrease GFR and worsen AKI. That being said, they can still be used in this disease and one must consider the clinical context. This is more problematic inpatients with atherosclerotic disease as opposed to FMD.
Renal artery angioplasty has not been shown to be superior to medical therapy in a meta-analysis published in the American Heart Journal in 2011. However, patients with refractory hypertension and flash pulmonary edema, may be considered for angioplasty. See this NEJM review on RAS for additional details.
Renal artery stenosis review
Wednesday, September 11, 2013
Thiamine and lactic acidosis
The approach to a lactic acidosis involves separating it into two possible classes, termed type A or type B. Type A is classified as lactic acidosis secondary to tissue hypoperfusion. This is usually not a mystery, and the patient has signs of end-organ damage, tissue hypoxia and hypotension. Type B lactic acidosis is felt to be from some derangement in metabolism of lactate, including:
 1. Drugs - metformin use, HIV medications (through mitochondrial dysfunction)
1. Drugs - metformin use, HIV medications (through mitochondrial dysfunction)
2. Alcohol - decrease gluconeogenesis
3. Malignancy - suspected to be secondary to increased cell turnover, though the mechanism is somewhat unclear, this is more often seen in leukemia/lymphoma
4. Type-D-lactate- present in patients with short gut syndrome, bowel bacteria (usually lactobacillis) produce D-lactate which is absorbed but cannot be metabolised by endogenous LDH leading to accumulation.
5. Liver dysfunction
Today we discussed a patient that had an elevated lactate of unclear cause. The patient was treated with thiamine with rapid improvement, which raises nutritional deficiency as a potential contributor to type B lactic acidosis.
Thiamine (vitamin B1) is a water soluble molecule that is required for aerobic metabolism. It acts as a cofactor for several enzymes present in the glycolysis pathway. Thiamine deficiency prevents pyruvate from converting into Acetyl-CoA, which is the entry point into the Krebs cycle, which is responsible for ATP production and NADH which is utilized in the electron transport chain. As a result, pyruvate accumulates and is metabolized into lactate resulting in acidosis.
Thiamine deficiency can occur quickly. Because it is water soluble it is not readily stored in fat and levels lowering the total body amounts. It is estimated that thiamine deficiency can develop as quickly as four weeks in poor nutritional intake. In cases of thiamine deficiency lactate production has been seen as early as 1-3 weeks. Risk factors for thiamine deficicency include, poor nutritional intake, folate deficiency, alcohol use, malabsorption, TPN and renal disease (specifically dialysis/peritoneal dialysis).
So next time you see a lactic acidosis in the absence of tissue hypoperfusion, consider vitamin B1 deficiency.
 1. Drugs - metformin use, HIV medications (through mitochondrial dysfunction)
1. Drugs - metformin use, HIV medications (through mitochondrial dysfunction)2. Alcohol - decrease gluconeogenesis
3. Malignancy - suspected to be secondary to increased cell turnover, though the mechanism is somewhat unclear, this is more often seen in leukemia/lymphoma
4. Type-D-lactate- present in patients with short gut syndrome, bowel bacteria (usually lactobacillis) produce D-lactate which is absorbed but cannot be metabolised by endogenous LDH leading to accumulation.
5. Liver dysfunction
Today we discussed a patient that had an elevated lactate of unclear cause. The patient was treated with thiamine with rapid improvement, which raises nutritional deficiency as a potential contributor to type B lactic acidosis.
Thiamine (vitamin B1) is a water soluble molecule that is required for aerobic metabolism. It acts as a cofactor for several enzymes present in the glycolysis pathway. Thiamine deficiency prevents pyruvate from converting into Acetyl-CoA, which is the entry point into the Krebs cycle, which is responsible for ATP production and NADH which is utilized in the electron transport chain. As a result, pyruvate accumulates and is metabolized into lactate resulting in acidosis.
Thiamine deficiency can occur quickly. Because it is water soluble it is not readily stored in fat and levels lowering the total body amounts. It is estimated that thiamine deficiency can develop as quickly as four weeks in poor nutritional intake. In cases of thiamine deficiency lactate production has been seen as early as 1-3 weeks. Risk factors for thiamine deficicency include, poor nutritional intake, folate deficiency, alcohol use, malabsorption, TPN and renal disease (specifically dialysis/peritoneal dialysis).
So next time you see a lactic acidosis in the absence of tissue hypoperfusion, consider vitamin B1 deficiency.
Tuesday, September 10, 2013
VBG versus ABG
The purpose of performing an arterial blood gas (ABG) is to identify alterations in acid base status or ventilation/oxygenation. Arterial blood gases can be troublesome at times. Patients may have hemodynamic instability (miking it tough to find the pulse), be unable to hold still for sample collection, and the procedure can occasionally be technically difficult. As a result, venous blood gases are often taken as a surrogate for arterial measures given they are easier to perform, less painful, and patients in the ICU often have central venous access.
There are several different areas from which a blood gas can be drawn:
1. Arterial blood gas - usually radial artery, though can be taken from brachial/femoral stab
2. Peripheral venous blood gas - any peripheral vein
3. Central venous gas - from right atrium, usually drawn from central line
4. Mixed venous gas - taken from the distal port of pulmonary arterial catheter
Of these different types of blood gas', the correlation with ABG's are variable:
1. Central venous vs ABG - pH 0.003-0.005 lower, pCO2 4-5 mmHg higher, no change in HCO3
2. Mixed venous vs ABG - similar to above
3. Peripheral venous vs ABG - pCO2 3-8 mmHg higher, HCO3 1-2 meq higher
pO2 values can not be reliably calculated from any form of venous gas.
Of these options central venous gas is preferred given its correlation with the ABG is most consistent and most studied, that being said, many patients dont have central lines.There was a study examining the effects of tourniquets on venous blood gas values and found that their use doesnt alter blood gas variables when used, which is reassuring.
The clinical context needs to be considered. If pateints are hypotensive and in shock, venous values are less reliable and ABG's are preferred. A study from NEJM in 1989 showed that as patients become more unstable, the arterial-venous difference gets larger. The difference between central venous and ABG pH in patients with shock was 0.1, highlighting that both measures need to be evaluated. They also concluded that tissue hypoperfusion is better assessed with central venous gas than ABG. Another NEJM study looked at a similar question in patients during cardiac arrest. The average ABG pH with during CPR was 7.41, while the mixed venous was 7.15! The pCO2 also changed dramatically. ABG may not reveal the true extent of tissue hypoxia compared to the mixed or central venous blood gas.
So, when in doubt, I say get an ABG, but in the critically ill patient/post CPR, a venous gas may show the true extent of the disease.
There are several different areas from which a blood gas can be drawn:
1. Arterial blood gas - usually radial artery, though can be taken from brachial/femoral stab
2. Peripheral venous blood gas - any peripheral vein
3. Central venous gas - from right atrium, usually drawn from central line
4. Mixed venous gas - taken from the distal port of pulmonary arterial catheter
Of these different types of blood gas', the correlation with ABG's are variable:
1. Central venous vs ABG - pH 0.003-0.005 lower, pCO2 4-5 mmHg higher, no change in HCO3
2. Mixed venous vs ABG - similar to above
3. Peripheral venous vs ABG - pCO2 3-8 mmHg higher, HCO3 1-2 meq higher
pO2 values can not be reliably calculated from any form of venous gas.
Of these options central venous gas is preferred given its correlation with the ABG is most consistent and most studied, that being said, many patients dont have central lines.There was a study examining the effects of tourniquets on venous blood gas values and found that their use doesnt alter blood gas variables when used, which is reassuring.
The clinical context needs to be considered. If pateints are hypotensive and in shock, venous values are less reliable and ABG's are preferred. A study from NEJM in 1989 showed that as patients become more unstable, the arterial-venous difference gets larger. The difference between central venous and ABG pH in patients with shock was 0.1, highlighting that both measures need to be evaluated. They also concluded that tissue hypoperfusion is better assessed with central venous gas than ABG. Another NEJM study looked at a similar question in patients during cardiac arrest. The average ABG pH with during CPR was 7.41, while the mixed venous was 7.15! The pCO2 also changed dramatically. ABG may not reveal the true extent of tissue hypoxia compared to the mixed or central venous blood gas.
So, when in doubt, I say get an ABG, but in the critically ill patient/post CPR, a venous gas may show the true extent of the disease.
Thursday, September 5, 2013
UTI prophylaxis
Recurrent urinary tract infections (UTI), defined as 2 or more infections in six months, or over three in a years period, is a frustrating disease for patients and physicians. This disease is common, where 20% of women presenting with a UTI will experience a recurrent episode at sometime. For the most part, these are new infections as opposed to persistence of the previous infection. When encountered, it raises the question as to whether patients should be treated with antibiotics on a more frequent basis in a prophylactic manner. Many women experience symptoms in relation to sexual activity, though in post-menopausal women, recurrent UTIs may have a different mechanism. Ageing results in changes in bladder function and anatomy, with increasing risk of prolapse. This can lead to urinary stasis and predisposition to recurrent UTIs. There are in general three approaches to this topic:
1. Continuous antibiotic prophylaxis
2. Post-coital prophylaxis
3. Intermittent therapy (not really preventative)
Choice of prophylactic strategy should be tailored to the individual patient. Cultures should be taken from the urine to confirm the presence of recurrent infection. Antibiotic choice should be based on culture and sensitivity results.
For women with recurrent UTI's, not in relation to coitus, continuous prophylaxis can be prescribed to patient as daily or three times weekly regiments. Usual antibiotics include: septra, nitrofurantoin, cephalexin, ciprofloxacin. Several studies have shown decreased rates of infection with this approach, and in 2004 a Cochrane review was published finding similar results. The number needed to treat in this review was 1.8 for one years therapy (which was the duration in the majority of studies). There was however increased side-effects in the therapy groups compared to placebo, where GI upset, rash and vaginal irritation was most common. In this study, there was no supreme antibiotics that performed better compared to others.
Following discontinuation of antibiotic therapy, women will tend to revert back to having recurrent UTI's. A reasonable approach is to trial antibiotics for 6 months, and after stopping see if the patient improves. Some individuals will have clusters of infection, which will be best treated with a period of drug followed by a holiday period. Longer prophylactic periods, upwards of two years, have also been advocated by some in the literature.
For females that develop infections in relation to sexual activity a post coital strategy can be taken, and has been found to have similar rates of success to continuous prophylaxis. One study randomized 135 women to daily prophylaxis with 125mg of cipro versus a post coital strategy. They found similar results between groups and a third of the amount of antibiotic used. A study published in JAMA 1990, comparing placebo to septra using a post-coital strategy showed a significant reduction in UTI's. Where 81% in the placebo group developed a recurrent UTI compared to 12.5% in the therapy arm.
Intermittent therapy that is self administered is a useful approach for patients who are compliant and motivated. This strategy results in a higher number of infections, given it is not a truly preventative approach, but symptomatic duration and total antibiotic dosing is minimized. One study testing this approach found that symptomatic episodes had a culture negative rate of 14%, suggesting that women were able to accurate identify the presence of a UTI based on symptoms in over 85% of cases.
With the ever-growing use of antibiotics, resistance to these medications is a concern, and should be considered in patients receiving chronic therapy. There have been studies of patients taking chronic antimicrobial therapy for UTI prophylaxis identifying breakthrough infections with bugs that were resistant to the antibiotic prescribed. Separate studies using septra and cipro found breakthrough infection rates with resistant organisms at a rate of 44% and 3% respectively. This high rate of septra resistant organisms in this group is concerning, and provides some evidence that its use as prophylaxis results in significant alterations in microbe susceptibility patterns. See this NEJM review from 1993.
Urinary tract infections NEJM
1. Continuous antibiotic prophylaxis
2. Post-coital prophylaxis
3. Intermittent therapy (not really preventative)
Choice of prophylactic strategy should be tailored to the individual patient. Cultures should be taken from the urine to confirm the presence of recurrent infection. Antibiotic choice should be based on culture and sensitivity results.
For women with recurrent UTI's, not in relation to coitus, continuous prophylaxis can be prescribed to patient as daily or three times weekly regiments. Usual antibiotics include: septra, nitrofurantoin, cephalexin, ciprofloxacin. Several studies have shown decreased rates of infection with this approach, and in 2004 a Cochrane review was published finding similar results. The number needed to treat in this review was 1.8 for one years therapy (which was the duration in the majority of studies). There was however increased side-effects in the therapy groups compared to placebo, where GI upset, rash and vaginal irritation was most common. In this study, there was no supreme antibiotics that performed better compared to others.
Following discontinuation of antibiotic therapy, women will tend to revert back to having recurrent UTI's. A reasonable approach is to trial antibiotics for 6 months, and after stopping see if the patient improves. Some individuals will have clusters of infection, which will be best treated with a period of drug followed by a holiday period. Longer prophylactic periods, upwards of two years, have also been advocated by some in the literature.
For females that develop infections in relation to sexual activity a post coital strategy can be taken, and has been found to have similar rates of success to continuous prophylaxis. One study randomized 135 women to daily prophylaxis with 125mg of cipro versus a post coital strategy. They found similar results between groups and a third of the amount of antibiotic used. A study published in JAMA 1990, comparing placebo to septra using a post-coital strategy showed a significant reduction in UTI's. Where 81% in the placebo group developed a recurrent UTI compared to 12.5% in the therapy arm.
Intermittent therapy that is self administered is a useful approach for patients who are compliant and motivated. This strategy results in a higher number of infections, given it is not a truly preventative approach, but symptomatic duration and total antibiotic dosing is minimized. One study testing this approach found that symptomatic episodes had a culture negative rate of 14%, suggesting that women were able to accurate identify the presence of a UTI based on symptoms in over 85% of cases.
With the ever-growing use of antibiotics, resistance to these medications is a concern, and should be considered in patients receiving chronic therapy. There have been studies of patients taking chronic antimicrobial therapy for UTI prophylaxis identifying breakthrough infections with bugs that were resistant to the antibiotic prescribed. Separate studies using septra and cipro found breakthrough infection rates with resistant organisms at a rate of 44% and 3% respectively. This high rate of septra resistant organisms in this group is concerning, and provides some evidence that its use as prophylaxis results in significant alterations in microbe susceptibility patterns. See this NEJM review from 1993.
Urinary tract infections NEJM
Wednesday, September 4, 2013
Metformin and Anemia
The list of causes of anemia are many, making the work up timely and at times invasive, where patients may require endoscopic procedures and occasionally bone marrow evaluation. B12 deficiency is a common problem and in itself has a long list of contributors, ranging from common (pernicious anemia) to zebra (nitrous oxide toxicity). The case of anemia discussed today involved a patient with DM2 who was taking metformin, which raised the question of metformin induced B12 deficiency. Given the widespread use of metformin in the treatment of diabetes, I felt a review of the literature surrounding this topic would be useful.
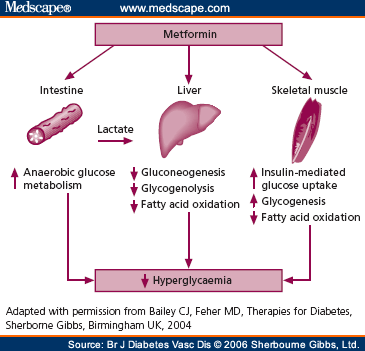 Metformin is from the biguanide class of oral hypoglycemics, which decrease hepatic gluconeogenesis and insulin dependant glucose utilization in peripheral tissues. It is supported as first line therapy for diabetes by the American Diabetes Association consensus guidelines and has become the most commonly used medication in this disease. Metformin results in decreased intestinal absorption in upwards of 30% of patients through a reduction in intestinal absorption. A randomized study involving nearly 400 patients randomized diabetics on insulin to metformin or placebo and monitored their B12, folate and homocysteine levels for 4 years. Compared to placebo, metformin resulted in decreased B12 levels and increased homocysteine levels. There was no difference in folate concentration when controlling for BMI and smoking. They felt that the number needed to harm was roughly 9 individuals per 4.3 years. B12 deficiency was found to be progressive, worsening over time in these patients where some did reach levels requiring supplementation.
Metformin is from the biguanide class of oral hypoglycemics, which decrease hepatic gluconeogenesis and insulin dependant glucose utilization in peripheral tissues. It is supported as first line therapy for diabetes by the American Diabetes Association consensus guidelines and has become the most commonly used medication in this disease. Metformin results in decreased intestinal absorption in upwards of 30% of patients through a reduction in intestinal absorption. A randomized study involving nearly 400 patients randomized diabetics on insulin to metformin or placebo and monitored their B12, folate and homocysteine levels for 4 years. Compared to placebo, metformin resulted in decreased B12 levels and increased homocysteine levels. There was no difference in folate concentration when controlling for BMI and smoking. They felt that the number needed to harm was roughly 9 individuals per 4.3 years. B12 deficiency was found to be progressive, worsening over time in these patients where some did reach levels requiring supplementation.
The mechanism was initially felt to be related to impaired bowel motility and subsequent bacterial overgrowth. However, B12 deficiency has been identified in the absence of bacterial overgrowth bringing this mechanism into question. Metformin is known to have activity against calcium absorption at the intestinal wall. As well, intrinsic factor absorption is calcium dependent, which raised the idea that calcium alterations may be involved in the mechanism of B12 deficiency. A study published in Diabetes Care looked at B12 levels in diabetics newly started on metformin. B12 levels dropped significantly after three months of therapy as did B12 precursors. Supplimentation with calcium led to an increase in B12 precursor within a month, with a non-significant increase in total B12 levels. There was no evidence of bacterial overgrowth in the study population as tested with hydrogen breath testing.
Rarely will the B12 deficiency associated with metformin result in hematologic or neurologic complications, though there are case reports of peripheral neuropathy from B12 deficiency. Neuropathy may be under diagnosed as well, given diabetic neuropathy presents in a similar fashion to B12 deficiency and is a common complaint amongst diabetics.
Below is the article linking calcium supplimentation and improved B12 levels.
B12 deficiency and metformin
 Metformin is from the biguanide class of oral hypoglycemics, which decrease hepatic gluconeogenesis and insulin dependant glucose utilization in peripheral tissues. It is supported as first line therapy for diabetes by the American Diabetes Association consensus guidelines and has become the most commonly used medication in this disease. Metformin results in decreased intestinal absorption in upwards of 30% of patients through a reduction in intestinal absorption. A randomized study involving nearly 400 patients randomized diabetics on insulin to metformin or placebo and monitored their B12, folate and homocysteine levels for 4 years. Compared to placebo, metformin resulted in decreased B12 levels and increased homocysteine levels. There was no difference in folate concentration when controlling for BMI and smoking. They felt that the number needed to harm was roughly 9 individuals per 4.3 years. B12 deficiency was found to be progressive, worsening over time in these patients where some did reach levels requiring supplementation.
Metformin is from the biguanide class of oral hypoglycemics, which decrease hepatic gluconeogenesis and insulin dependant glucose utilization in peripheral tissues. It is supported as first line therapy for diabetes by the American Diabetes Association consensus guidelines and has become the most commonly used medication in this disease. Metformin results in decreased intestinal absorption in upwards of 30% of patients through a reduction in intestinal absorption. A randomized study involving nearly 400 patients randomized diabetics on insulin to metformin or placebo and monitored their B12, folate and homocysteine levels for 4 years. Compared to placebo, metformin resulted in decreased B12 levels and increased homocysteine levels. There was no difference in folate concentration when controlling for BMI and smoking. They felt that the number needed to harm was roughly 9 individuals per 4.3 years. B12 deficiency was found to be progressive, worsening over time in these patients where some did reach levels requiring supplementation.The mechanism was initially felt to be related to impaired bowel motility and subsequent bacterial overgrowth. However, B12 deficiency has been identified in the absence of bacterial overgrowth bringing this mechanism into question. Metformin is known to have activity against calcium absorption at the intestinal wall. As well, intrinsic factor absorption is calcium dependent, which raised the idea that calcium alterations may be involved in the mechanism of B12 deficiency. A study published in Diabetes Care looked at B12 levels in diabetics newly started on metformin. B12 levels dropped significantly after three months of therapy as did B12 precursors. Supplimentation with calcium led to an increase in B12 precursor within a month, with a non-significant increase in total B12 levels. There was no evidence of bacterial overgrowth in the study population as tested with hydrogen breath testing.
Rarely will the B12 deficiency associated with metformin result in hematologic or neurologic complications, though there are case reports of peripheral neuropathy from B12 deficiency. Neuropathy may be under diagnosed as well, given diabetic neuropathy presents in a similar fashion to B12 deficiency and is a common complaint amongst diabetics.
Below is the article linking calcium supplimentation and improved B12 levels.
B12 deficiency and metformin
Subscribe to:
Comments
(
Atom
)

